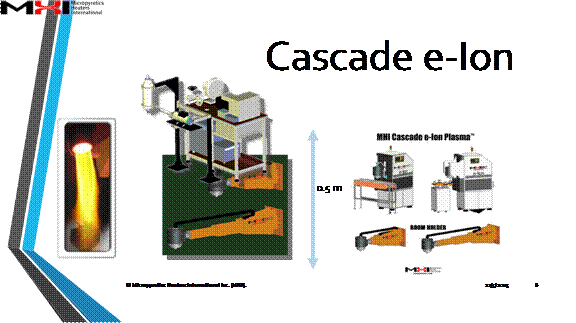
What is Cascade e-Ion Plasma™?
The World's First Stable Thermal Cascade Plasma Generator
Now available for Surface Heat Treating, Functionalization of Polymers
About Cascade e-Ion Technology
The Cascade e-Ion technology is presented in the form of an easy to use, easy to teach yourself plasma machine. The break through process produces high watt atmospheric plasma capable of rapidly cleaning, deburring, depositing, growing, hardening, joining, reacting of gaseous to metallic materials and many more applications like rapid brazing, the most rapid heat treatment and ultra low cost of total energy used.
When used for surface treating XPS and other fine electronic probes display the fine (nano or surface quantum dot) structure, depth of ionic penetration and many undiscovered new phase formations which may be used advantageously for applications.
Customer Testimonial. "I had a look at your Table comparing the cost and various features of (standard RF) plasma nitriding and MHI (Cascade) e-ion plasma nitriding machines and the advantages of the later are remarkable. This type of comparison ......... an alluring proposition for any stakeholder to purchase such an equipment".
Cascade e-Ion Plasma™ technology uses electricity and just air (or any other gas) to produce a CleanElectricFlame® plume that contains ions, electrons, radiation and hot gas. It is a versatile tool for several possible applications. To the best of our knowledge this is the only plasma generator that offers close to 100% power transfer - thus making it a unique high-efficiency directed energy source. Applications could included formation of a single layer of atoms (or stacks), with far-reaching potential. Known as two-dimensional materials, this class has grown within the past few years to include lattice-like layers of carbon (graphene), boron (borophene), hexagonal boron nitride (white graphene), silicon (silicene), phosphorous (phosphorene) and 2d oxycarbonitrides or specian carbon nitrogen compounds. Check our Cascade e-Ion applications page.
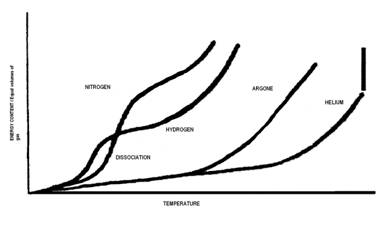
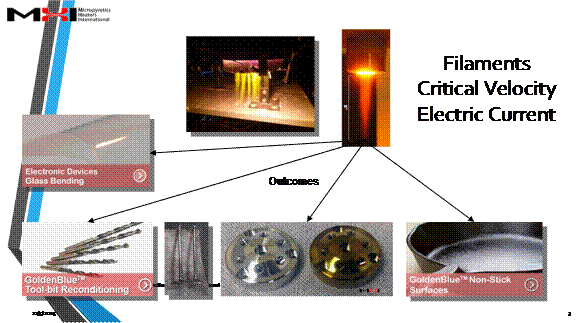
On account of the number of ions quickly available to a part (please see pdf below) the Cascade e-Ion plasma™ finds uses in machine-tool development of new and reconditioning machine tools, non-stick surfaces, clean glass bending, covers for liquid metals, plastics (polymerization and functionalization), metals (heat treatment of semiconductors, metal finishing, nano-deposition and brazing and related operations). The machines also find use in ceramics (ceramic surface modification, nano-treatment and other modern materials from new process type use). Please see pdf below or click through to our Cascade e-Ion applications page for typical solutions. Shown below are the first ionization energy of gases as a function of the atomic number.
Plasma Review
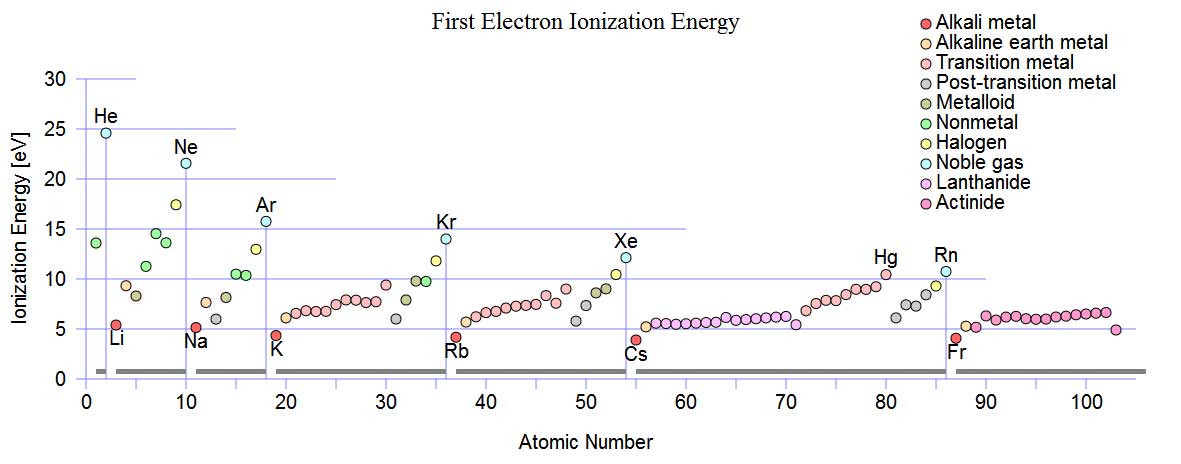
Source: http://en.wikipedia.org/wiki/File:First_Ionization_Energy.svg
Case studies for applications are shown below. Please also click to applications
How do the Cascade Ions interact with a surface
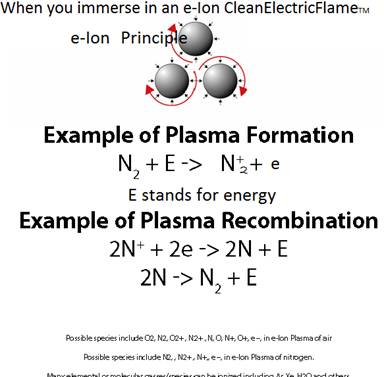
The Cascade e-ion can be used for almost any gas (element or molecule). Air, N2, Forming Gas, Xenon, Argon are some of the common gases that are used with the Cascade e-Ion Machines. How much heat is available depends on many factors. Air, containing 79%N2, 20% O2 and rest the common ingredients can be plazmized. Ionization of molecules often leads to changes in molecular geometry. Only details about the first ionization energy of a non- vibratory molecule are shown below. An air CleanElectricFlame™ is shown on the picture on the left, in the picture above. The ionization energy is slightly different between atoms and molecules of the same element. The issue of ionization is complex for molecules which posses vibration and rotational energies. Ionization energy reports for molecules may involve some approximations. Ionization energy is reported in eV (eV/atom) or kJ/mol. The first ionization energy for hydrogen is 13.894eV (1312.0 kJ/mol). The first ionization energy is 12.5eV and 13.61eV for an oxygen molecule and oxygen atom respectively (for KJ/mole please read below). The first ionization energy is 15.6eV and 14.45eV for a nitrogen atom and nitrogen molecule respectively. The First Ionization Energy as a function of the atomic number is shown above. The First Ionization Energy of an element (atom) is the energy needed to remove the outermost electron from a neutral atom in the gas phase. When recombination with an electron occurs for the ionized atom, tremendous heat is generated. Thus rapid heating of a surface/substrate is the result. The e-Ion is able to transfer the ionic species and cause recombination to occur where the heat and electric field is required. The thermal cascade plasma is a remarkable scientific achievement. A graphic of the heating potential is shown in the illustration below for activated ionization. The last two reactions are the recombination reactions that produce intense localized energy release, often in the form of heat. The e-Ion Cascade Thermal Plasma® is a unique patented device that can produce a CleanElectricFlame™. Although Nitrogen is used for the example almost all gases can be used. When ionized the chemical behavior of the atom is influenced. The electronic structure is shown below:
N: 1s2 2s2 2p3 and O: 1s22s2 2p4
N+: 1s2 2s2 2p2 and O+: 1s22s2 2p3
In general, the first ionization energy increases as we go from left to right across a row of the periodic table. The first ionization energy decreases as we go down a column of the periodic table. Note from the ionization vs. atomic number chart above that within a periodic table row that contains N2 and O2 there is small a change in the ionization trend. In the ionization chart above, note that there is a small drop in energy deposited to surface with oxygen ions compared to nitrogen ions. Hund's rule predicts that the three electrons in the 2p orbitals of a nitrogen atom all have the same spin, but electrons are paired in one of the 2p orbitals on an oxygen atom (thus creating an opposite spin pair) and thus explaining the ionization energy difference between N2 and O2. Please contact MHI for references to thermal calculations or directly view published article for heat transfer for plasma. This particular linked article explains how even a slight amount of e-ion plasmazation is capable of vast heat transfer rate improvements when compared to heating by other methods like convection or radiation alone. Molecular Ionization: The first ionization energy of H2 are different than the atom It could be more or less depending on the bond strength (energy). The measured ionization energy of H2 is 1488 kJ mol​-1. Note that comparison to the ionization energy of a hydrogen atom, is 1312 kJ mol​-1. More energy is required energy to remove an electron for the hydrogen molecule ionization, when compared to the hydrogen atom. The Gibbs free energy of an ionic reaction could thus be different in final reaction results depending on species. In general molecular ionization is a much more complex process than atomic ionization because of the availability of many states.
Ask MHI for steam-ion, and other gas mixtures using the automated MHI SIMGAS. Plasma Functionalization.
The surfaces that are produced because of the reaction of the cascade e-ion with them depends on the gas used. Many of the examples discussed in the case studies section are with ionized air. Surface colors are influenced by many properties including the composition of nitrides and oxynitrides and wavelengths used. The refractive index and extinction coefficient are often very sensitive to processing conditions. Some discussion on solar cell use is given here.
- Customize for deposition type and shape. Contact MHI.
- Compare price and energy efficiency with ordinary transferred arc or induction plasma deposition or laser.
No electrodes to change in cascade plasmas. - Improve Surfaces from Tool bits, Non-stick to Electrical. Titanium Oxynitride-Nitride, Yittria Zirconia (with dopants), Boron Carbide, Silicon Carbide, Molybdenum diSilicide.
- Super-ionic bending and Ionic Surface Hardened Glass and Nano Surface Depositions.
- WC and other high wear resistant surfaces for tool bits.
- Tubes and complex shapes. Thick Alumina on Aluminum. In situ nitriding of tubes for dies.
- Easily make functionally graded coatings.
- Custom Turnkey Solutions with Complete Electronic Controls. Increase endurance of stamping and hot working dies,
- Highly stable e-Ion cascade thermal plasma™
- New ionic low-cost surface treating and deposition. Minimize pollution and minimize noise - improve energy efficiency.
- Titanium Oxynitride Nitride coatings on HSS and other surfaces. Easy oxynitride or nitride nitride formers are Ti, V, Zr, Al, Mn, Mo,Fr, Cr and many others. In addition various eta phases carbon-niitrides and borides are possible.
- Non-Stick high hardness metallic surfaces
- MHI provides coating for Nickel Aluminide formation with the cascade e ion. Contact MHI.
-
Petrochemical: reforming furnaces, cracking furnaces, pyrolysis and fired heaters,
- Clean aluminum production.
- Clean Coal Combustion and Ignition (reduced aromatics).
- VOC cleaning.
-
Steel and Heat treatment: radiant tubes, furnace rolls
-
Glass making industry: Roll barrels for float glass line
- Color of surfaces. Contact MHI for Edax, Microstructure and XPS results where available.
Some plastic surface molecules may be reoriented (or functionalized) with a plasma and sometimes made into antimicrobial surfaces for resisting some type of bacterial colonization’s or biofilm growth? Please Contact MHI for ongoing detailed early investigation with an EPA CRADA alliance. Review some recent results using the De-e-Ion nanodeposition possibilities for such applications for biofilm elimination on metals even after 7 years of use in biofilm forming environments.
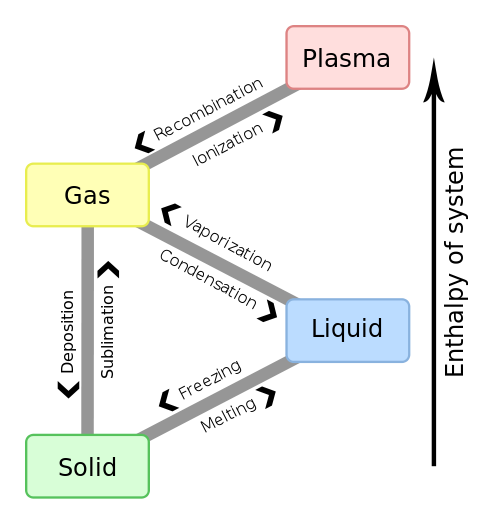
Figures that explain ionization (partial credit Wikipedia https://en.wikipedia.org/wiki/Fermionic_condensate#/media/File:Phase_change_-_en.svg)