Introduction to Electric Steam-generators That Offer High Energy Efficiency and High Steam Temperatures.
Modern Steam Generators are easy to operate. Set the flow and temperature on the touchscreen HMI, then press start. It’s that simple. Models range from 1 kg/h to several thousand kg/h for the adjustable steam-flow rate.
Video: OAB Unit 360 view (download).
What is Steam? In engineering, steam is vaporized water, a chemically pure, invisible gas that exceeds 100 degrees Celsius at standard atmospheric pressure. It occupies about 1,600 times the volume of the same mass of liquid water and can reach even higher temperatures as pressure increases, forming high-temperature or superheated steam.
Recent innovations in steam technologies include electric decarbonized steam systems that enable rapid production and adjustments without pressurization, allowing for steam temperatures of up to 1200°C at a variable set rate within the performance limits of a steam generator. Electrical steam generator- systems generally feature a touchscreen for controlling the steam flow rate, temperature, and power settings, with programmable operation for high energy efficiency.
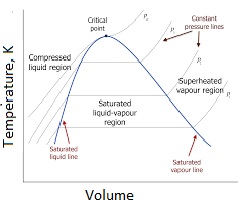
Steam is a valuable energy source because water has a high heat of vaporization. At sea level and one atmosphere of pressure (101 kPa or 1 bar), steam boils at about 100°C (212°F). The boiling point varies with pressure and, therefore, with location. Unlike pressure-typical boilers, the temperature of the steam gas from a steam generator is independent of pressure, allowing steam production at any temperature. Water’s boiling point increases with pressure, and beyond 647.096K and 22.064 MPa, it exists in a supercritical state. Steam can be either saturated or superheated. Saturated steam boilers produce steam at the pressure-dependent boiling temperature.
The output from old-style pressure boilers often contains water droplets mixed with gas, a condition called wet steam. Dry steam, or superheated steam gas, is easily generated by electric steam generators at temperatures above the boiling point with no water droplets (also known as high-quality steam). Dry steam is typically required for antimicrobial, chemical, and power applications.
Switching from fuel-based boilers to electric boilers can significantly reduce CO2 emissions. For example, replacing steam turbines with electric drivers in chemical and petrochemical plants can lead to substantial CO2 emission reductions, which is further enhanced by the energy efficiencies and other control features of electric steam generators.
Choose temperatures between Saturated and 1000C.
For high, back-pressure requests
Steam generators are flexible. One can set the required steam flow rate, output temperature, and back pressure. They also provide pulsing and power settings for energy efficiency. Infinite flow variation is available across a wide range. Some typical variable flow ranges are listed below – however please note that other requested parameters will influence this range. Please request a quote for your specific requirements.
The variable flow range will change with the requested maximum temperature.
|
Steam and Water Vapor Terminologies:
What is humidity? Humidity is a measure of the amount of water vapor present in the air. There are several ways to understand Humidity (generally used only below 100°C, at 1 atmosphere). Water vapor has limited solubility in air below 100 °C (and 1 bar pressure).
What is dew point? The dew point is the temperature at which air becomes saturated with water vapor and can no longer hold more water in its gaseous form. Some water vapor condenses into liquid water when the air is cooled below its dew point. The dew point temperature is a measure of humidity. The dew point is the temperature the air needs to be cooled to (at constant pressure) to achieve a relative humidity (RH) of 100%.
What is Relative Humidity? The Relative Humidity (RH) compares the amount of water vapor in the air-steam mix to the amount in the air if the air were fully saturated. It is a ratio that compares the amount of water vapor in the air with the amount of water vapor present at saturation. Relative Humidity is a percentage of water vapor expressed as a percent of saturation. The saturation amount increases with temperature. Humidity is a contextual property. Below the saturation temperature, Relative Humidity (RH) is a term that is used when Steam is mixed with air. Relative Humidity is a ratio that compares the amount of water vapor in the air with the amount of water vapor present at saturation for equivalent conditions. It is a percentage: water vapor is expressed as a percent of saturation. At 30 °C (86 °F), air volume can contain up to ~4 percent water vapor. However, at -40 °C (-40 °F), it can hold no more than 0.2 percent. The equations for water content, partial pressure, and the enthalpy of moist air are given in the adjacent column.
What is Specific Humidity? At high temperatures above the boiling point, it is more appropriate to talk about Specific Humidity (also called the humidity ratio), which is the mass of water vapor present in a unit mass of dry air; that is, it is the ratio of the mass of water vapor to the mass of dry air. This can range from 0 to 1. For example, at 25°C and one atmospheric pressure, if the Specific Humidity is 0.02 (it means two grams of water vapor in 100 grams of dry air). Note that the corresponding Relative Humidity (RH) is ~10%.
Brief History of Steam Generation
| Period | Nature of Device | Who developed it first? | |
| Late 1700s –Early 1800s (about 0.5-2.0 lbs. of CO2 per 3414 BTU of heat energy from fossil fuels)
| Combustion steam boilers. Typically, saturated steam with water droplets. | These were the earliest boilers. Source Wiki: Stirling Boiler Company, Ohio, SA. Later merged with Babcock and Wilcox. | |
| Late 1900’s (about 0.5-2.0 lbs,, of CO2 per 3414 BTU of heat energy from fossil fuels)
| Higher pressure. Combustion-fired and some electric boilers. Typically, saturated steam. | Several worldwide manufacturers. Steam generation required high-pressure boilers and was bulky. | |
| 2015 to the present Zero NOx, Zero CO2, Zero CO, Zero particulates. A Deep Decarbonization Product.
| Electric high-temperature. Steam generators 1000°C and higher Instant St am. Variable f ow. Variable press re. Variable temperature. Steam above the inversion temperature. | MHI Inc. USA. Compact, Instant, and Reliable. Video: OAB Unit 360 view (download) Controlling steam processes and humidity is easy. The instant steam generators directly sense humidity signals to regulate the amount of water conversion. |
Modern, high-quality superheated Steam is employed for several critical applications. High-temperature Steam is helpful for pharmaceutical, biopharmaceutical, comfort, utility, and chemical uses because of a lack of water droplets. Noncondensing superheated Steam is the most energy-efficient method of steam use. Employing dry superheated Steam above the inversion temperature of Steam leads to the best antimicrobial and most rapid drying steam applications. The wicking properties and oxygen control of this type of Steam are attractive features of high-temperature steam. High-temperature steam availability leads to high-productivity applications. Steam generator products are classified into two types, namely:
- MightySteam® Pure Steam Products (Industrial and R&D Use)
- MigthySteam® Controlled Humidity Products (Commercial Use)
- The average molecular weight of air is approximately 29g, heavier than the molecular weight of steam (H2O), which has a molecular weight of 18g. However, the actual densities of air and steam vary greatly based on steam-to-air ratio, temperature, and pressure. For example, at 10 bar, air is heavier than steam at temperatures under 162 °C (~325 ºF) but lighter than steam at temperatures over that. This is sometimes called density inversion temperature, which is vital for drying. The relationship flips again at 20 bar. Electric steam generators do not need high pressure, making process development much easier.
What is superheated Steam? Steam can be saturated or superheated. When at the boiling temperature (which depends on the pressure), the type of Steam is called saturated st am. Saturated Steam is often a mixture of water and gas. When above the boiling temperature, it is called superheated Steam, especially when water droplets are no longer present (i.e., dry or high-quality Steam). At sea level and one-atmosphere pressure (101 kPa), Steam boils at ~100°C (~212°F) at 1-atmosphere pressure at sea level, and the saturated steam temperature for this press at 1-bar, ~100°C temperature. The boiling point changes with pressure and with geographical location. The Steam gas can be superheated (called steam gas). Steam generators make superheated Steam-gas. Unlike what is required for boilers, the steam-gas temperature is unrelated to the pressure. Steam at any temperature and any unrelated pressure to Tsat can thus be requested from a steam generator.
Modern steam generators use sophisticated electric and computer controls that permit flow rate and temperature variations. This versatility makes them suitable for various applications, from chemical reactions, dry cleaning, and antimicrobial use to drying ores and wet materials, including food. Modern steam generators offer significant benefits in downstream efficiencies and dryness. Pressurizing steam to obtain a higher temperature and using it at room pressure involves a considerable loss in energy efficiency.
What is Supercritical Steam: There’s a unique mix of temperature and pressure – called the critical point – where the difference between liquid and gas ceases to exist. This happens at 374 °C (705 °F) and 218 Bar for water. This type of steam forms beyond the critical point (>647.096K and >22.064 M a). About ~30% of free monomeric H2O molecules exist at the critical point. The rest contain different types of H2 bonds. Supercritical water has very low surface tension with its gas or liquid phase; therefore, no interfaces can delineate the liquid/gas interface. Above 647.096K, increasing the pressure cannot liquefy supercritical Steam. There is no difference in the energy content between super-critical steam at 460◦C and 250 bar and medium-pressure steam at 280°C and 10 bar-i.e., 3.0 GJ/ton. But their entropies are different. Steam at 280°Cand 10-bar has an entropy of 7.0 kJ/kg-K. The super-critical steam at 460◦C and 250 bar has an entropy of 5.7 kJ/kg-K. Now, it is clear why supercritical steam is used to generate power. However, this has little bearing on the thermal use of steam for reactions or cleaning purposes. Electric steam generators prevent such energy losses. Supercritical steam can capture CO2.
Another example of a supercritical material is Supercritical CO2 (above 304.13 K and 7.38 M a). CO2 in this state is used to decaffeinate coffee beans. Its viscosity and diffusivity are like a gas, penetrating the beans quickly. However, its density is like that of a liquid. It binds to caffeine (this property is much more critical than its supercritical fluid properties). Supercritical CO2 is also used in dry cleaning.
The properties of superheated Steam and types of steam kinetics are shown on the Steam Calculator page.
Take me to the Airtorch® Models Page Take me to the Steam Generator Models Page.
A pound of water x 0.016 = cubic feet at 62.2 °F.
A pound of water x 0.12 = gallons of water
1 gallon of H2O liquid = 8.33 lb. @ 62.2 degrees Fahrenheit. The mineral content of calcium and magnesium in the water determines the hardness of water.
The freezing temperature of water at one Bar is 32 Fahrenheit (°F) = 0 Celsius (°C) (this phase change temperature is only mildly pressure dependent)
Water specifications pertinent to Boilers and Steam Generators.
Soft and Hard Water:
Any boiler feedwater treatment system usually incorporates the following water purification methods:
- Filtration.
- Softening.
- Reverse osmosis (RO). This water is generally recommended for modern steam generators. In 2025, the average operating cost for a 100 kg/h per hour (~100 LPH) reverse osmosis machine typically ranged from $150 to $300 per year for light-to-moderate usage (approximately 10 hours per day). The capital cost for a 100 LPH was about $500 approximately. Please contact the various manufacturers for current information.
- Primary ion exchange.
- Deaeration or Degasification.
Feedwater is piped to a steam generator to form continuous high-temperature dry steam. The condensate may be combined with treated makeup water and re-fed.
Water treatment plants today are very cost efficient and inexpensive.
Softening. Water softening removes hardness due to calcium and magnesium in the water. A softening resin accomplishes this, typically with a strong acidic resin, effectively capturing and removing hardness ions from the stream.
Makeup Water Intake. Replacement or make-up water is drawn from treated city supplies or raw water treatment systems. Steam generators can use the condensate return. The water supplied to a steam generator must meet the specifications of RO and DI water.
Filtration: Water is typically filtered to remove sediment, turbidity, and organic material. Membrane filtration units may be the most cost-effective pretreatment option.
Deaeration or Degasification Following all other treatment steps, the makeup water and condensate from the boiler system are combined, removed, and gasified to prevent corrosion.
- Water Hardness Ratings:
The water’s calcium and magnesium content determines its hardness. Water softness is called Grains/gallons or Parts/Million (ppm). - Less than 1 GPG or less than 17.1 ppm is soft
1.0 to 3.5 GPG or 17.2 to 60 ppm is slightly hard
3.6 to 7.0 GPG or 61 to 120 ppm is moderately hard
7.1 to 10.5GPG or 121 to 180 ppm is hard
10.6 GPG & over 181 ppm & over is very hard
| Electrical Properties | Resistivity at 20°C (ohm-m) | Conductivity at 20°C (S/m). S is Siemens. One micromho is equal to one microsiemens. |
| Ultrapure Water | 1.82×109 | 5.49×10−10 |
| Sea Water | 2.1×10−1 | 4.8 |
| Tap Water | 2×101 to 2×103 | 5×10−4 to 5×10−2 |
| Air | 109 to 1015 | 10−15 to 10−9 |
Grades of water.
DI water. DI-grade water is purified with almost all its mineral ions removed, such as cations like sodium, calcium, iron, and copper, and anions like chloride and sulfate. The DI process leverages specially manufactured ion-exchange resins that exchange hydrogen (H+) and hydroxyl (OH-) ions for dissolved minerals and then recombine to form water (H O). Primary Ion Exchange – Deionizers may be used instead of membrane filtration for large volumes of water or high-pressure boilers. Ion exchange typically produces water of comparatively higher quality and resistivity and provides better yields. The conductivity is about 1 to 5 micro-mho/cm.
Reverse Osmosis (RO) cleans tap water to make it roughly 90% to 99% pure. Deionization (DI) filters exchange positive hydrogen and negative hydroxyl molecules for positive and negative contaminant molecules in water. DI filtering and other processes are sometimes called “water polishing.” RO can remove bacteria, salts, organics, silica, and hardness. RO and nanofiltration both employ membrane filtration to capture contaminants. RO systems for industrial purposes typically provide a 65 to 75 percent recovery rate; a very efficient water use RO results in exceptionally pure water. The conductivity is typically 5-25 micro-mho/cm
Please take me to the Airtorch® Models Page Take me to the Steam Generator Models Page.
Typical water feed requirements for ultraclean steam generation.
- RO and DI water or highly purified water. 0.1 – 50 micro-mho/com.
- Free of Amines, Chlorine, and Chlorides
- Silica less than one ppm
- Total suspended solids (TDS) – low. Total dissolved solids (TDS) combine the sum of all ion particles smaller than about 2 microns. This includes all the disassociated electrolytes that make up salinity concentrations and other compounds such as dissolved organic matter. Total dissolved solids are reported in mg/L or ppm. An empirical TDS-“constant” relates TDS (ppm) and water conductivity in micro-mho/cm’. The conductivity of a water sample can be multiplied by the TDS-constant for an approximate TDS. A TDS constant of 0.55-0.7 is reasonable for typically clean (i.e, free of urea and organic waste) RO, DI, or tap water. However, if the water source is known to be high in calcium or sulfate ions, a constant of 0.8 may be used.
- Total hardness less than 1ppm
- For conductivity, see the table above.
Does high pressure enable the outcome of Steam or a chemical reaction? The short answer is seldom significantly above 10 °C. For the long answer, click here.
High-quality superheated Steam has applications in drying, cooking, proper and complete bacterial inactivation, fracking, chemical processes engineering, comfort heating, chemical processes, fuel production, tablet making, mixing, and materials processing High-temperature Steam in one atmosphere packs the proper punch required for these applications while minimizing the dangers of using high-pressure boilers This type of superheated Steam is used in applications requiring a critical need to reduce the processing time Superheated Steam often offers a higher heat transfer coefficient and high enthalpy content, enabling many unique applications When at a high temperature, significantly above the inversion temperature, such Steam is often considered a non-toxic antimicrobial agent Superheated Steam at high temperatures also offers superior reactions, for example, in energy reactions such as bio-fuels, reforming, hydrogen production, ammonia production, and denaturing, all with rapid heat transfer kinetics In the US, more than 90% of electric power is produced using Steam as a working fluid, mainly by steam turbines Condensation of Steam to water occurs downstream, but such wet-steam conditions must be carefully controlled to avoid excessive blade erosion and preserve energy efficiency Oxidation and erosion tests can be done with Steam Generators There is no moisture from the start-up in many modern steam generators The HGAs and OAB produce high-quality pure Steam The HGA-M is for applications requiring steam-gas(air) mixtures The Mightysteam® and SaniZap® models are helpful for steam cleaning at several levels of cleaning The OAB® and GHGA models are used for industrial and R&D purposes The high-temperature Steam also tends to be useful for pharmaceutical, biopharmaceutical, comfort industry, utility, and chemical uses because of a lack of droplets and rapid antimicrobial action for several orders of log reductions in a short duration.
The standard steam diagrams for process design are below.
The gases in the atmosphere exert a certain amount of pressure (about 1013 millibars at sea level). Vapor pressure measures the air’s water vapor content using the partial pressure of the water vapor in the air (Pressure may be expressed using a variety of units: pascals, millibars, pounds per square inch (psi), among others). Since water vapor is one of the gases in the air, it contributes to the total air pressure. The contribution by water vapor is relatively small since it only makes up a few percent of the total mass of a parcel of air. The vapor pressure of the water in the air at sea level, at a temperature of 20oC, is ~24 mbar at saturation (about 3% by volume).
Does the saturation pressure of H2O in the air depend on the total pressure? Yes, as the total pressure of a system decreases, the Relative Humidity will decrease. Likewise, as the total pressure of a system increases, the Relative Humidity will increase until saturation is reached.
Suppose 10 grams of water vapor were present in each kilogram o. dry air, and should the air at that temperature be saturated at 30 grams of water vapor per kilogram of dry air, the Relative Humidity is 10/30=33 3%. A parcel of air at sea level, at a temperature of 25oC, would be completely saturated if there were ~20 grams of water vapor in every kilogram of dry air. We use a mixing ratio: grams of water vapor per kilogram of fully saturated air. If this air contained 20 grams of water vapor per kilogram of dry air, we would say that the Relative Humidity is 100%.
What is its relative humidity if a parcel of air (at sea level and 25ooC) had 10 grams of water vapor per kilogram of dry air? Answer: The Relative Humidity would be 0%. Ten grams of water vapor/kg dry air compared to the maximum possible 20 grams of water vapor/kg dry air is 10/20= 0%. If the parcel of air (at sea level and 25oC) had 18 grams of water vapor per kilogram of dry air, what is its Relative Humidity? The Relative Humidity would be 18/20= 0%. In the examples above, the temperature is taken to be about 2 °C. Another more technical term is the ratio of the actual vapor pressure to the saturation vapor press re. You will note below that the HGA-M produces a lot of Steam with very high specific humidity because above 100C in one atmosphere, air and Steam mix well as any other “ideal gasses could” Below 100C, the RH is an essential limitation on how much water vapor can mix with air For one-atmosphere condition, above 100C, one should use the term specific humidity, which is the mass of water vapor (i.e., Steam) ratio when mixed with a unit mass of dry air.
Water saturation pressure at a temperature(total pressure 1 atmosphere) (This chart is helpful for comfort heating) | ||
| Temperature, °C | Bar | Psi |
| 2 | 0.007 | 0.1024 |
| 4 | 0.008 | 0.1180 |
| 10 | 0.012 | 0.1781 |
| 14 | 0.016 | 0.2319 |
| 20 | 0.023 | 0.3393 |
| 25 | 0.031 | 0.4598 |
| 30 | 0.042 | 0.616 |
| 34 | 0.053 | 0.7723 |
| 40 | 0.073 | 1.0711 |
| 50 | 0.12 | 1.7915 |
| 60 | 0.20 | 2.8929 |
| 70 | 0.31 | 4.5253 |
| 80 | 0.47 | 6.8768 |
| 90 | 0.693 | 10.179 |
| 96 | 0.87 | 12.730 |
| 100 | 1.00 | 14.710 |
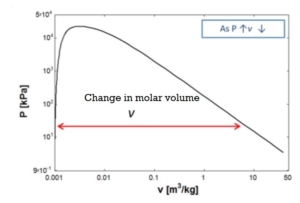
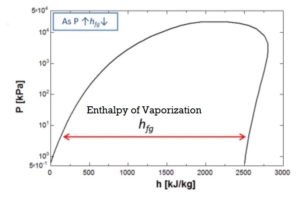
The enthalpy of vaporization and the molar volume change fall with increasing pressure from 1 Bar to 10 Bar.
Power Steam Jet

SaniZap-600-4-240. A must for cleaning. (Mobile Platform)
Discover Steam Tunnels for Continuous use
 |
Sometimes one requires a steam-gas mixture instead of pure st am. The HGA-M-01 and HGA-M-04 can enable this.
Calculating your parameters.
Endotoxins, microbes, and bacteria are known to be inactivated by heat and H2O. All MHI superheated steam productions produce high-temperature Steam that, during protection, encounters temperatures over 5 0C. Several models’ steam output temperature and humidity conditions are controllable, as discussed below. Refer to the HGA-M manual to see how your HGA-M is configured/rated. The mass fraction of Steam in the final flow is about 18% for a valve setting, which gives, for example, 200ml/15.5 minutes (i.e., speciHumiditydity is about 2 %). We are assuming that the water vapor is ideal and that the enthalpy of the water vapor in the air can be taken to be the enthalpy of saturated vapor at the same temperature (~ 2501.3+ 1.82 T (kJ/kg)) Temperature, T, is in units of degrees centigrade. Of course, after a specific temperature and pressure ~374C and 22.06 MPa called the critical temperature Tc and critical pressure Pc, respectively, no amount of pressure can cause condensation. The superheated steam generator can produce a steam-air temperature over this temperature, but the output is not at critical conditions because the pressure is lower!
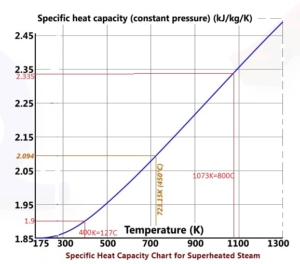
The HGA and OAB operate at close to 100% power efficiency from which the steam temperature can be calculated by the equation below for the efficiency of HGA-M, h is enthalpy and T, the temperature is given in Kelvin (Kelvin=273+Centigrade):
![]()
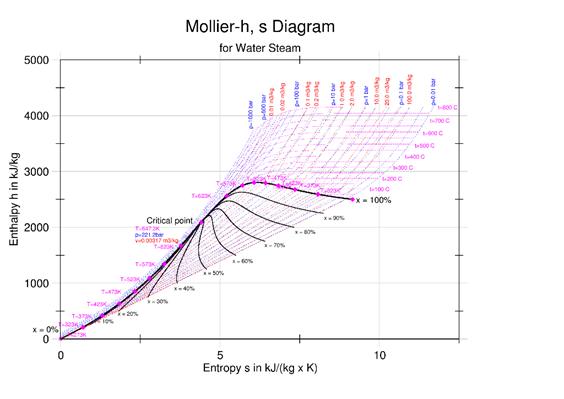
h (enthalpy per kg) of Steam is obtained from the figures above at 1-atmosphere pressure, steam tables, or the Mollier diagram Or; you can measure the temperature from the exit thermocouple.
GENERAL INSTRUCTIONS FOR HGA-M
Instructions on how to use and protect the HGA-M are enclosed with the prod ct. It’s a unique, designed device with ease of use. Turn on the air and monitor with a flow meter so the SCFM does not fall below .4. Higher airflows give lower temperatures, or you may control power with a separately obtained controller. Then turn on the heater and finally open the water metering valve. Steam-air will be the product. Remember to read the manual for the shutdown procedure.
Q: When do you dry with Steam instead of hot air? Answer- Steam has many benefits. The main one is the availability of a gas with a lot of stored enthalpy at a lower temperature than the corresponding dry air with the same enthalpy. So, isupposeyou are interested in drying paper with an ignition temperature of 450F,. In that case, using superheated steam at a much lower temperature may produce the same drying efficiency as hot air at a higher temperature, which could be more than the paper ignition temperature.
Follow all safety procedures. NOTE STEAM IS AN ODORLESS GAS AT VERY HIGH TEMPERATURES. STEAM, LIKE OTHER HOT GASSES, WILL BURN OUT. STEAM PACKS A LARGE AMOUNT OF ENTHALPY SO THAT THE BURN COULD BE SEVERE; DO NOT ALLOW THE STEAM TO FALL ON THE SKIN. WEAR GOGGLES AND GLOVES And Protection for your clothes, ALWAYS.
A selection of steam tables is given below. It is best to use a standard text or the International Association for the Properties of Water and Steam (IAPWS)
If you want superheated, high-quality Steam, you should now search for the HGA or OAB models that address your needs. The information below refers to the HGA-M models only where an air or gas-steam mixture is required. You may e co. Humidity is a design term or property used when using air or gas mixtures with steam.
IT IS EASY TO BE CONFUSED BETWEEN RELATIVE AND SPECIFIC HUMIDITY. RELATIVE HUMIDITY DEPENDS ON TEMPERATURE; SPECIFIC HUMIDITY ONLY RELATED TO THE MASS FRACTION.
See below from the public site http://www.pals.iastate.edu/mteor/mt206/lectures/feb7/tsld020.htm.
Potential uses: For layering, epoxy drying, and other film use, superheated Steam is required at one atmospheric pressure. This makes it ideal for steam drying or steam oxidation. Attempt use also for precipitating crystals of several small sizes, including nanocrystals from solutions. Precipitation droplet sizes may be controlled by controlling the cooling rate, impingement conditions, and surface type.
The steam temperature depends on the water valve setting and air inflow setting.
HGA-M (typical settings at Full Power):
- Air 1.45 CFM (inlet at ~30C) and water 330ml in 45 mins (inlet at ~30C) yield a steam-air temperature of about 350°C.
- Air 1.4CFM (inlet at ~30C) and water 200ml in 20 mins (inlet at ~30C) yield a steam-air temperature of about 250°C.
- Air 1.8CFM (inlet at ~30C) and water 200ml in 20 mins (inlet at ~30C) yield a steam-air temperature of about 150°C.
The graph below gives a fair idea of adjusting the HGA750-1 for different specific humidity levels. As the specific humidity increases, there is a corresponding decrease in overall temperature as total energy is consumed. The gas thermocouple is correct as the exit for the graph below the st am. If you try to reduce the steam gas temperature too much, you may not be able to get superheated Steam, and instead, a heated mist may be the output product. The red line graph requires a power controller.
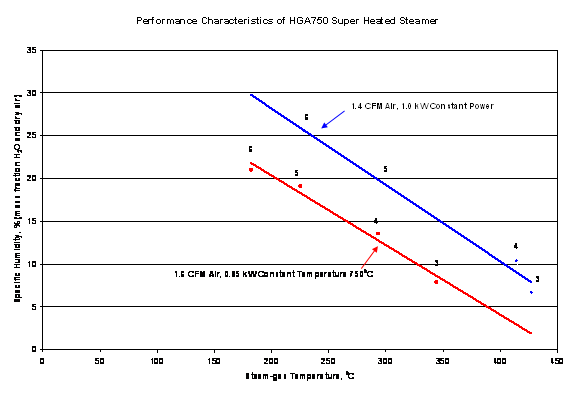
Your results may vary. The values above should be considered approximate because of the placement of the thermocouple, restrictions on flows, and other random errors usually present in multivariate measurements. The user must optimize all valve settings to get the best results for specific applications.
SUPERHEATED STEAM IS AN ODORLESS GAS (not to be confused with mist). Output: constant steam air (superheated steam). Safety precautions must be taken when dealing with hot gases.
DO NOT USE UNITS WITH COMBUSTIBLE LIQUIDS; THE DANGER OF SUPERHEATED STEAM SHOULD BE WELL UNDERSTOOD. PLEASE WEAR GLOVES, GLASSES, AND A HARD AT. PROTECTIVE CLOTHING IS REQUIRED; STEAM CAN PENETRATE CLOTHES.
The product uses cleaning technologies, drying technologies, curing technologies, and nanotechnologies.
Patents issued applied and are pending for the HGA.
A control thermocouple for the hot air generator part is included. Steam output temperature thermocouples and brackets are sold separately, or users may provide their own. This unit requires an electrical 110-120V 50/60Hz supply. The 1KW system requires compressed air input.
RELATIVE HUMIDITY DEPENDS ON TEMPERATURE; SPECIFIC HUMIDITY IS RELATED TO THE MASS O LY. IT IS EASY TO BE CONFUSED BETWEEN RELATIVE AND SPECIFIC HUMID TY. See below from http://www.pals.iastate.edu/mteor/mt206/lectures/feb7/tsld020.htm.
 |  |
The evolution to clean steam processes for performance and energy efficiency. | |
Old Style Pressure Boilers. Often cause emissions of GHGs as more often than not they are powered by fossil fuel combustion. Old-style boilers have low automation, which means that the temperature and flow rate cannot be varied or set on a display panel. Most old-style boilers require idling, whereas new-era electric steam generators can be switched on and off at will. | High Efficiency. Electric Steam Generators. Select the flow rate and temperature. Choose remote on-off and pulsing, and choose back pressure. No idling – optimize energy. Low piping requirements. |
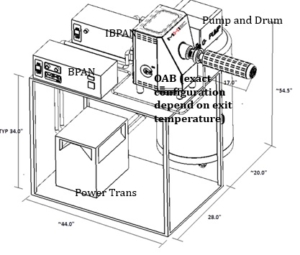
OAB® (Electric Steam Generator)
Summary page for types of electric steam generators
How to quickly treat with antibacterial steam/air (set residence time)
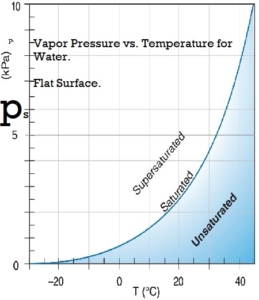
Water vapor pressure
Water vapor will be in the space above a closed container partly filled with water. The concentration of water vapor depends only on temperature. It is not dependent on the amount of water and is only slightly influenced by the air pressure in the container. The water vapor exerts pressure on the container’s walls. The empirical equations below give a good approximation of the saturation water vapor pressure at temperatures within the limits of the earth’s climate.
Saturation vapor pressure, ps, in pascals:
ps = 610.94 *exp(( T*17.6294) / ( T + 243 ) )
where T is the temperature in degrees Celsius
The svp below freezing can be corrected after using the equation above, thus:
ps ice = -4.86 + 0.855*ps + 0.000244*ps2
The next formula gives a direct result for the saturation vapor pressure over ice:
ps ice = 611.2 exp( 22.587*T / ( T+273)
The Pascal (Pa) is the SI unit of pressure = newtons / m2. Atmospheric pressure is about 100,000 Pa (standard atmospheric pressure is 101,300 Pa).
Water vapor concentration
The relationship between vapor pressure and concentration is defined for any gas by the equation: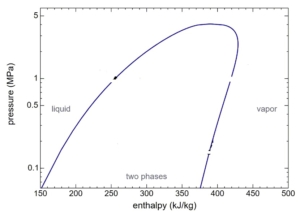
p = nRT/V
p is the pressure in Pa, V is the volume in cubic meters, T is the temperature (degrees Celsius + 273.16), n is the quantity of gas expressed in molar mass ( 0.018 kg in the case of water ), R is the gas constant: 8.31 Joules/mol.K
To convert the water vapor pressure to concentration in kg/m3: ( Kg / 0.018 ) / V = p / RT
kg/m3 = 0.002166 *p / ( T+ 273.16 ) where p is the actual vapor pressure
Relative Humidity
Relative Humidity (RH) is the ratio of the actual water vapor pressure to the saturation water vapor pressure at the prevailing temperature.
RH = p/ps
RH is usually expressed as a percentage rather than as a fraction.
The RH is a ratio. It does not define the air’s water content unless the temperature is given. The RH is widely used in conservation because most organic materials have an equilibrium water content that is mainly determined by the RH and only slightly influenced by temperature.
Notice that air is not involved in the definition of RH. Airless space can have an RH. Air transports water vapor in the atmosphere and air conditioning systems, so the phrase “RH of the air” is commonly used and only occasionally misleading. The independence of RH from atmospheric pressure is not essential on the ground. Still, it does have some relevance to calculations concerning the air transport of works of art and conservation by freeze-drying.
The Dew Point
The water vapor content of air is often quoted as a dew point. This is the temperature to which the air must be cooled before dew condenses from it. At this temperature, the actual water vapor content of the air is equal to the saturation water vapor pressure. The dew point is usually calculated from the RH. The first one calculates ps, the saturation vapor pressure at the ambient temperature. The actual water vapor pressure, pa, is:
pa= ps * RH% / 100
The next step is to calculate the temperature at which pa would be the saturation vapor pressure. This means running backward the equation given above for deriving saturation vapor pressure from temperature:
Let w = ln ( pa/ 610.78 )
Dew point = w *238.3 / ( 17.294 – w )
This calculation is often used to judge the probability of condensation on windows and within walls and roofs of humidified buildings.
The dew point can also be measured directly by cooling a mirror until it fogs. The RH is then given by the ratio
RH = 100 * ps (dewpoint)/ps (ambient)
The concentration of water vapor in the air
It is sometimes convenient to quote water vapor concentration as kg/kg of dry air. This is used in air conditioning calculations and is quoted on psychrometric charts. The following calculations for water vapor concentration in air apply at ground level.
Dry air has a molar mass of 0.029 kg. It is denser than water vapor, which has a molar mass of 0.018 kg. Therefore, humid air is lighter than dry air. If the total atmospheric pressure is P and the water vapor pressure is p, the partial pressure of the dry air component is P – p. The weight ratio of the two components, water vapor and dry air, is:
kg water vapor / kg dry air = 0.018 *p / ( 0.029 *(P – p ) )
= 0.62 *p / (P – p )
At room temperature P – p is nearly equal to P, which at ground level is close to 100,000 Pa, so, approximately:
kg water vapor / kg dry air = 0.62 *10-5 *p
Thermal properties of damp air
The heat content, usually called the enthalpy of air, rises with increasing water content. This hidden heat, called latent heat by air conditioning engineers, has to be supplied or removed to change the relative humidity of the air, even at a constant temperature. This is relevant to conservators. The transfer of heat from an air stream to a wet surface, which releases water vapor to the airstream at the same time as it cools it, is the basis for psychrometry and many other microclimatic phenomena. Control of heat transfer can be used to control the drying and wetting of materials during conservation treatment.
Air at zero degrees Celsius is defined to have zero enthalpy. The enthalpy, h, in kJ/kg, at any temperature, T (K), between 0 and 60°C is approximately:
h = 1.007T – 0.026, below zero: h = 1.005T
The enthalpy of liquid water is also sometimes defined as zero at zero degrees Celsius. At the same temperature, turning liquid water to vapor requires a considerable amount of heat energy: ~2501 kJ/kg at 0C.
At a temperature T the heat content of water vapor is:
hw = 2501 + 1.84T
Once generated, water vapor also requires more heat than dry air to raise its temperature further: 1.84 kJ/kg.K compared to about 1 kJ/kg.K for dry air.
The enthalpy of moist air, in kJ/kg, is, therefore:
h = (1.007*T – 0.026) + g*(2501 + 1.84*T)
g is the water content (specific humidity) in kg/kg of dry air
The Psychrometer
The final formula in this collection is the psychrometric equation. The psychrometer is the nearest to an absolute method of measuring RH that the conservator ever needs. It is more reliable than electronic devices because it depends on the calibration of thermometers or temperature sensors, which are much more reliable than electrical RH sensors. The only limitation to the psychrometer is that it is difficult to use in confined spaces (not because it needs to be whirled around but because it releases water vapor).
The psychrometer, or wet and dry bulb thermometer, responds to the RH of the air in this way:
Unsaturated air evaporates water from the wet wick. The heat required to evaporate the water into the air stream is taken from the air stream, which cools when it contacts the wet surface, thus cooling the thermometer beneath it. An equilibrium wet surface temperature is reached, which is roughly halfway between ambient temperature and the dew point temperature.
The air’s potential to absorb water is proportional to the difference between the mole fraction, ma, of water vapor in the ambient air and the mole fraction, mw, of water vapor in the saturated air at the wet surface. It is this capacity to carry away water vapor that drives the temperature down to, Tw, the wet thermometer temperature, from the ambient temperature ta :
(mw – ma) = B(Ta- Tw)
B is a constant whose numerical value can be derived theoretically by some rather complicated physics.
The water vapor concentration is expressed here as a mole fraction in air rather than vapor pressure. Air is involved in the psychrometric equation because it brings the heat required to evaporate water from the wet surface. Therefore, The constant B depends on total air pressure, P. However, the mole fraction, m, is simply the ratio of vapor pressure p to total pressure P: p/P. The air pressure is the same for both ambient air and air in contact with the wet surface, so the constant B can be modified to a new value, A, which incorporates the pressure, allowing the molar fractions to be replaced by the corresponding vapor pressures:
pw – pa= A* ( Ta- Tw)
The relative humidity (as already defined) is the ratio of pa, the actual water vapor pressure of the air, tops, and the saturation water vapor pressure at ambient temperature.
RH% = 100 *pa/ ps = 100 *( pw – ( Ta- Tw) * 63) / ps
When the wet thermometer is frozen, the constant changes to 56
The psychrometric constant is taken from R.G. Wylie and T. Lalas, “Accurate psychrometer coefficients for wet and ice-covered cylinders in laminar transverse air streams,” in Moisture and Humidity 1985, published by the Instrument Society of America, pp. 37 – 56. These values are slightly lower than those in general use. Tables and slide rules are available for calculating RH from the psychrometer, but a programmable calculator is convenient for this job. Alternatively, click on the calculator.
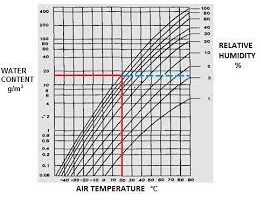
One of the most extensive uses of industrial heating is in the steam generator sector. High-temperature steam is used in comfort heating, sanitation, controlled drying of textiles, consumer packing (shrink packaging), curing concrete, soy production, antimicrobial uses, food processing, food safety, meat tenderizing, milk processing, laundry, skincare, pyrolysis-gasification of municipal waste and other areas. Industrial systems use a considerable amount of steam, not all of which can be recycled. In the past, steam was made by fossil fuel-heated pressure boilers, which are typically inefficient and produce water droplet-laden saturated steam, implying a significant loss in energy and water management efficiencies.
Relative Humidity Chart
High-Temperature Steam Reactions
CO2+4H2 ⇌ CH4+2H2O, ΔrH0 = (-)165kJ per mole CO2
CO2+H2 ⇌ CO+H2O, ΔrH0 = 41kJ per mole CO2
CO+3H2 ⇌ CH4+H2O, ΔrH0 = (-) 206kJ per mole CO
Best Thermal Battery